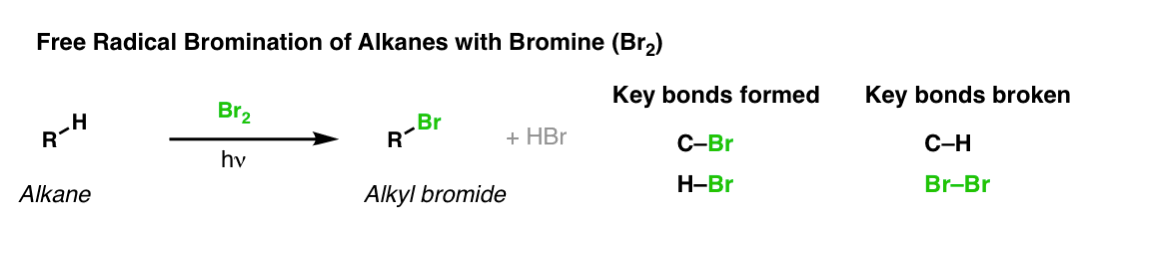
Description: When treated with bromine (Br2) and light (hν) alkanes are converted into alkyl bromides. In the absence of any double bonds, with Br2 this is selective for tertiary carbons.
Notes: Light (hν) is the initiator for this reaction. Alternatively, sometimes peroxides (RO-OR) can serve as radical initiators along with heat.
Examples:
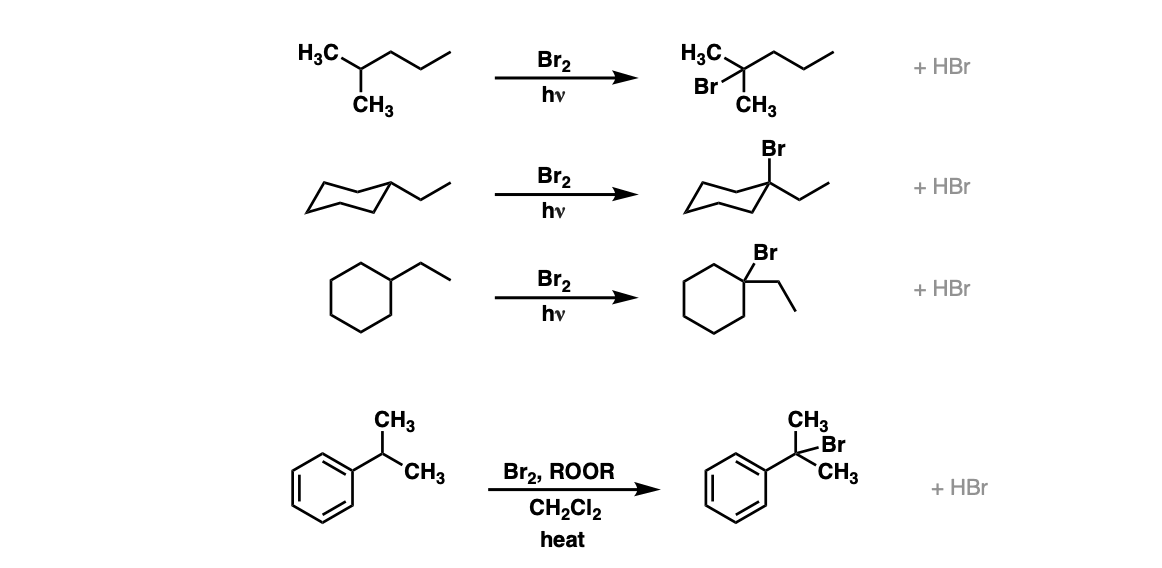
Notes: Note that each of these reactions produces HBr as a byproduct. If this reaction occurs at a stereocenter, a mixture of alkyl bromides will be obtained. In the fourth example, ROOR represents peroxides, with a weak O-O bond which is ruptured homolytically by heat (Δ). CH2Cl2 is the solvent here.
This table allows for the relative comparison of the selectivity of radical chlorination and bromination (reaction rates with typical primary, secondary, tertiary alkyl C-H bonds)
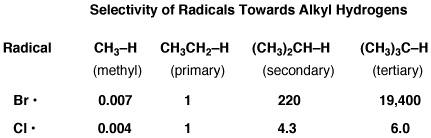
The key takeaway here is that bromine radicals are almost 20,000 times more likely to remove a hydrogen from a tertiary C-H bond than from a primary C-H bond.
Mechanism: When treated with light, Br2 fragments to bromine radicals (Step 1, arrow A). At any given time only a small amount of these radicals is present. There’s lots of leftover Br2! Remember this as it comes up in step 3. Bromine radical then abstracts a hydrogen from the tertiary carbon, leaving behind the tertiary radical (Step 2, arrows B and C). The tertiary radical then reacts with Br2, giving the tertiary bromide (Step 3, arrows D and E).
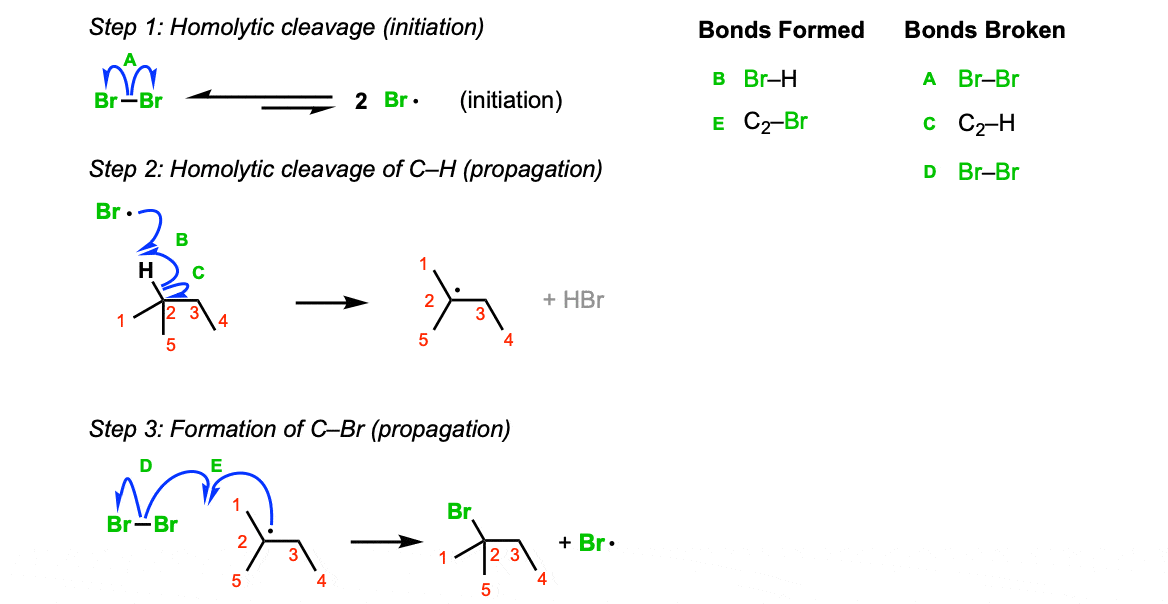
Notes: Avoid the common mistake of having the radical react with Br• in the third step – that’s not a propagation reaction, that’s termination!
Note that tertiary radicals are more stable than secondary or primary radicals and thus require the least energy to form (another way of saying this is that tertiary C-H bonds are weaker than secondary and primary C-H bonds). The higher selectivity of this reaction for tertiary C-H (when compared with chlorination) is a reflection of the fact that formation of the weaker H–Br bond provides less of a driving force for this reaction as compared to H–Cl (which is a stronger bond).
Additional examples:
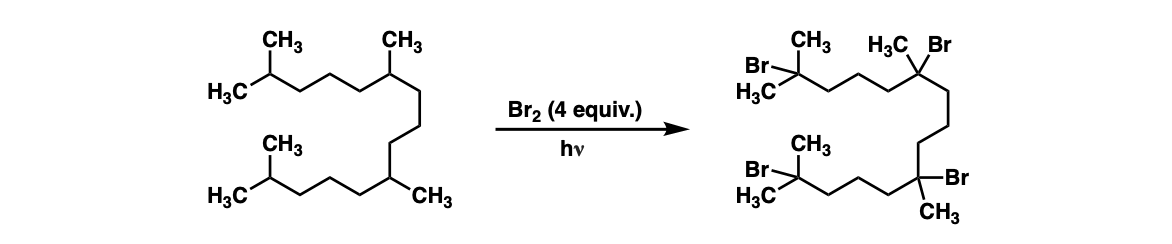
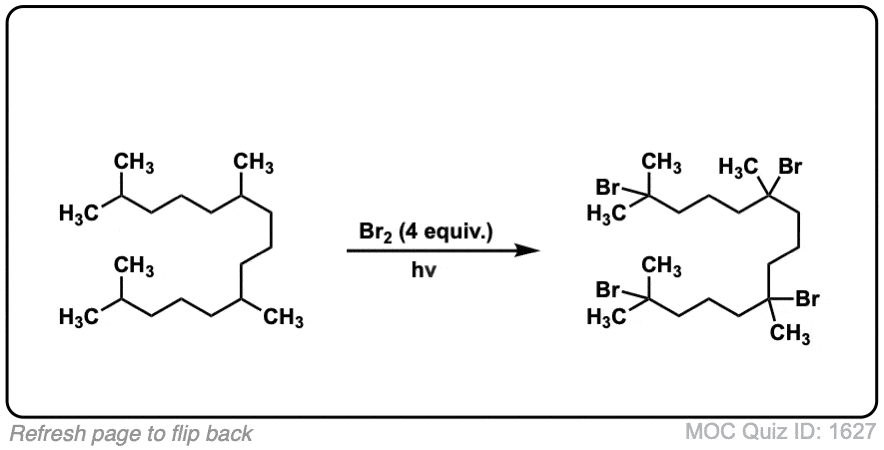
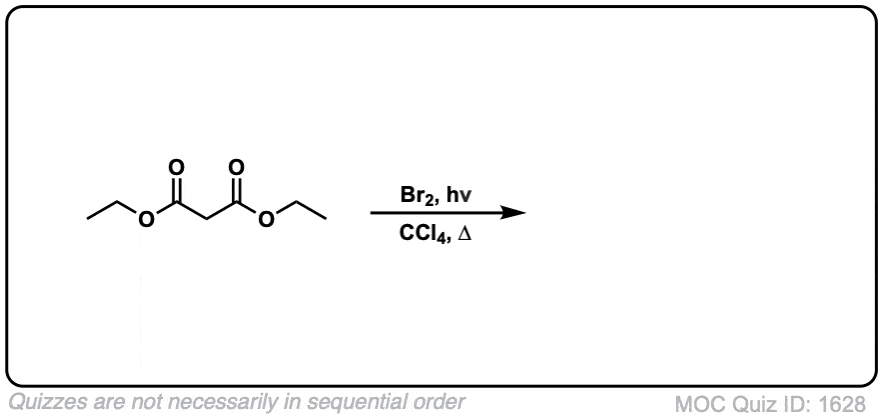
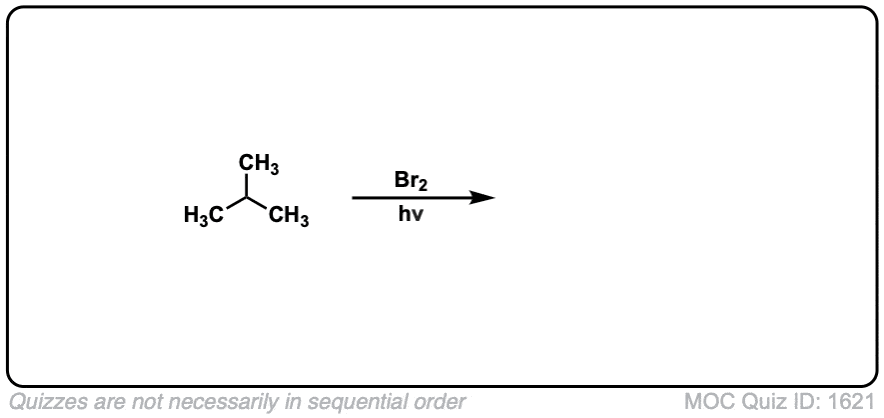
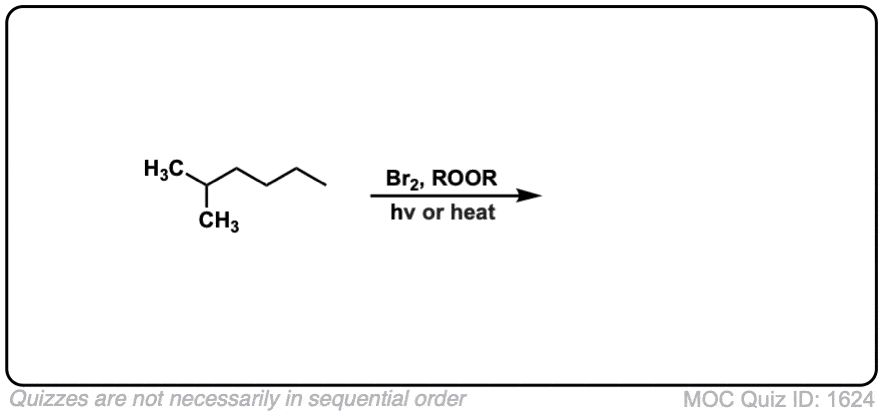
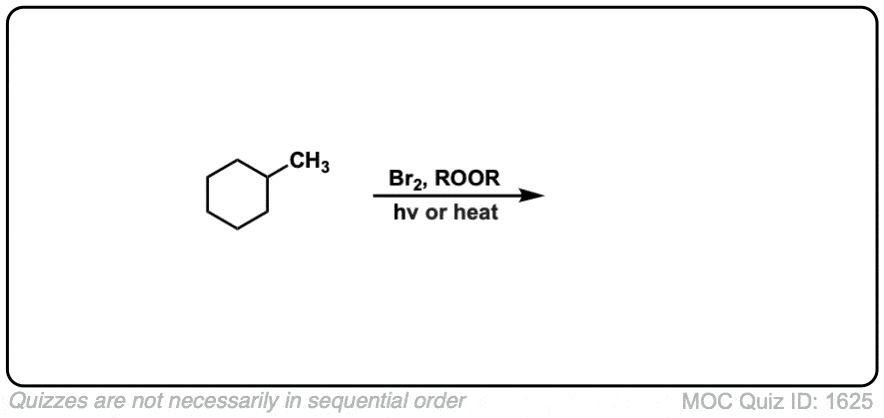
Quiz Yourself:
 Click to Flip
Click to Flip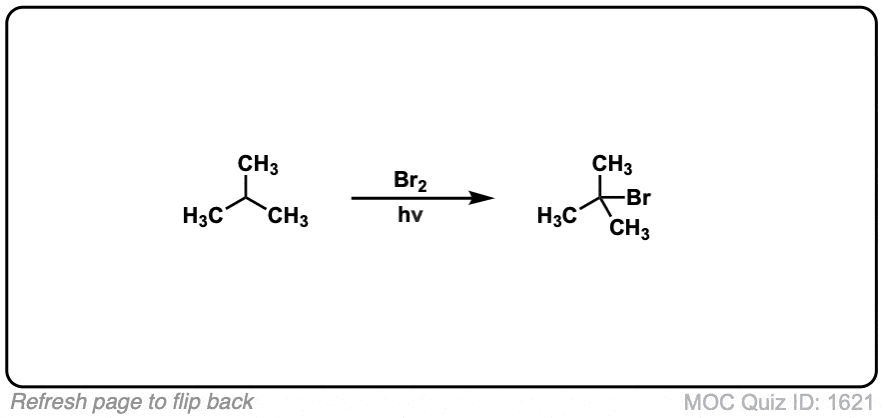
 Click to Flip
Click to Flip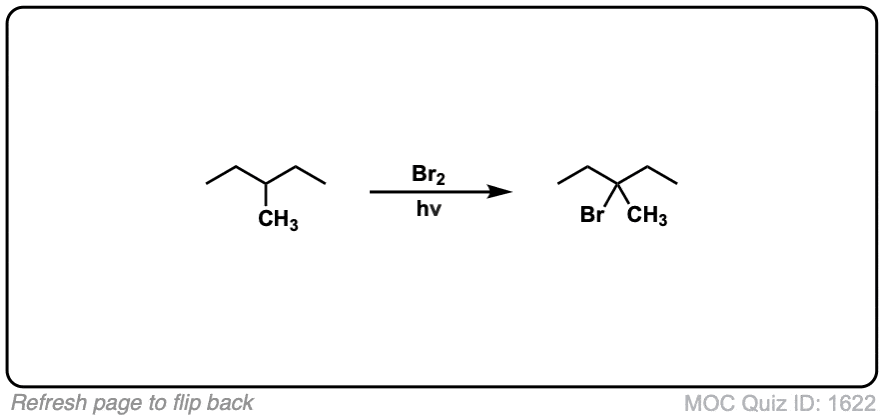
 Click to Flip
Click to Flip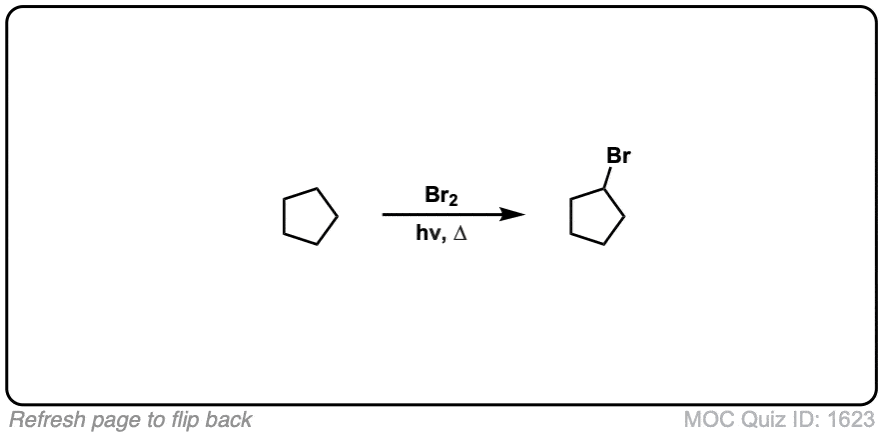
 Click to Flip
Click to Flip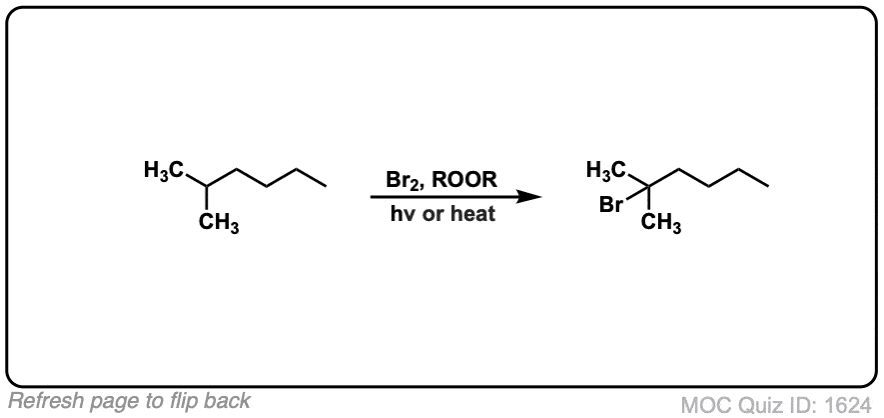
 Click to Flip
Click to Flip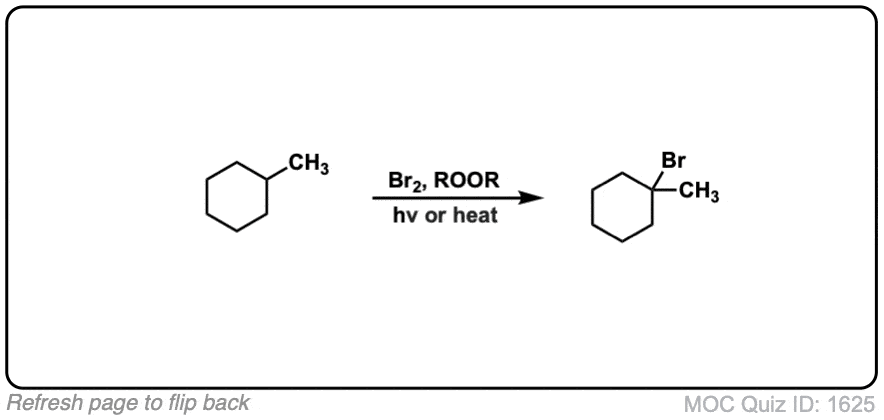
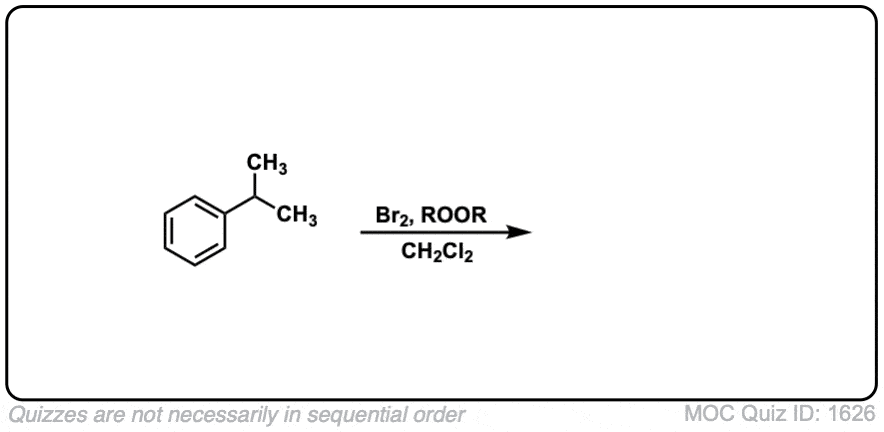 Click to Flip
Click to Flip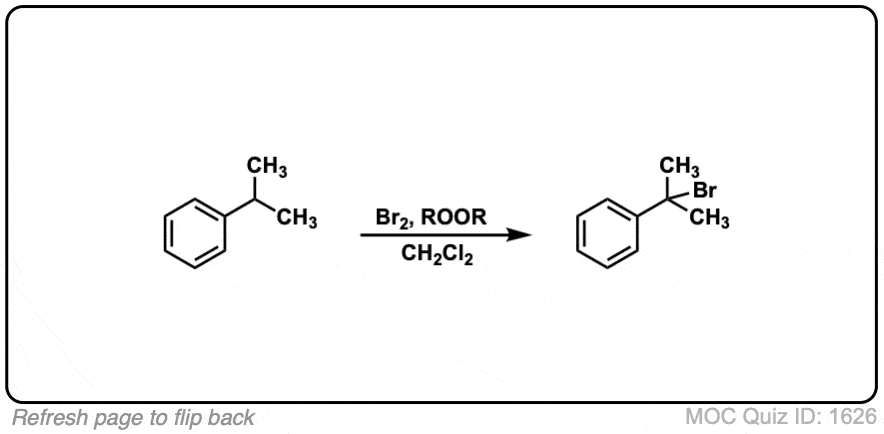
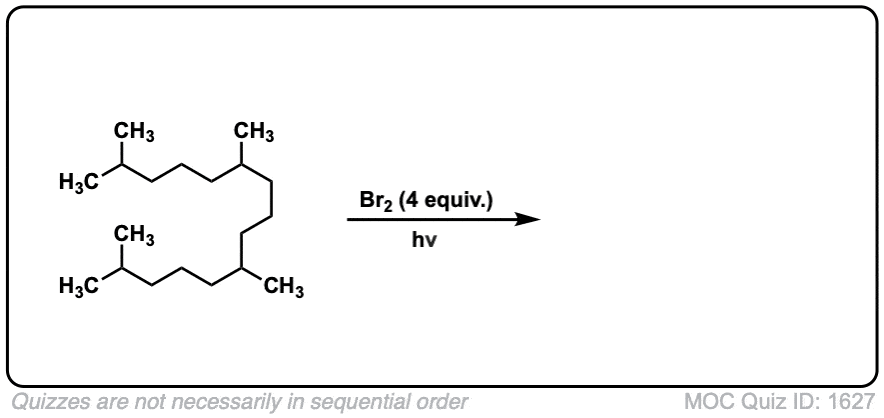 Click to Flip
Click to Flip